The Ministry of Health (MoH) alerted the public to a health product and two cosmetic products tested by the Laboratory of Pharmacy Section, Department of Scientific Services, Ministry of Health and found to be adulterated with undeclared, potent western medicine.
The health product is Majun Resdung manufactured by CT Aleys found containing dexamethasone, while the cosmetic products are Fairy Skin Glowing Facial Set – Glowing Facial Toner and Skin Magical Rejuvenating Set 1 – Rejuvenating Cream No 1 manufactured in the Philippines found containing hydroquinone.
The adulterants in the products can cause adverse effects and are potentially hazardous to consumers.
Unsupervised long-term consumption of potent corticosteroids such as dexamethasone can cause increase blood glucose levels leading to diabetes, high blood pressure, cataracts, muscular and bone disorders, and an increased risk of infections. Long-term use of corticosteroids can also lead to Cushing’s syndrome which is characterised by a round face or ‘moon face’, and upper body obesity with thin limbs. Consumers who have taken high doses of steroids over a prolonged period may suffer from withdrawal symptoms which include fatigue, muscle and joint pain, fever, low blood sugar, low blood pressure and dehydration.
Hydroquinone is used in western prescription medicines to treat skin conditions and is prohibited in skincare cosmetic products under the Medicines (Cosmetic Products) Regulations. Unsupervised use of hydroquinone may cause skin hypersensitivity, skin discolouration resulting in gradual darkening of the affected skin area and an increased risk of skin cancer.
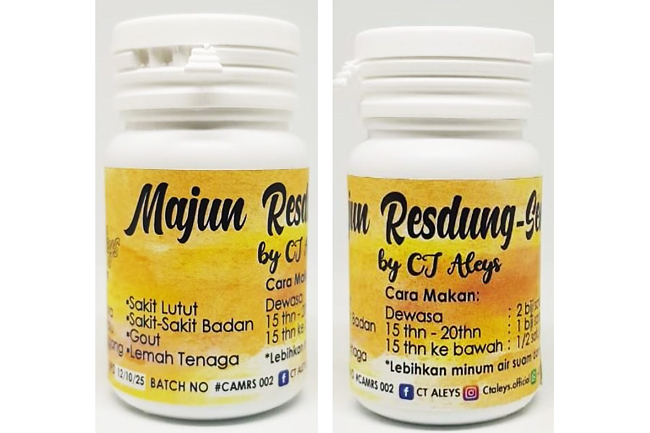


The MoH has not issued any approval for the importation for the sale of these products and/or Cosmetic Product Notification Acknowledgement Letter for the sale of the rest of affected products. Following these findings, the products are not allowed to be imported and sold in the country.
Those who purchased or used the products are advised to stop using them immediately and consult a medical practitioner if unwell or experience any undesirable reactions as a result of using them.
Those involved in the retail of these products (including online retail such as through Facebook) are reminded that it is an offence under the Poisons Act 1956 to sell any product containing any substance controlled under the said Act. The penalty for such an offence upon conviction, is a fine of BND8,000 or six months’ imprisonment. If a person commits an act that amounts to such a degree of negligence so as to endanger or be likely to endanger human life, then the person is guilty of an offence which carries a penalty of a BND16,000 fine of and 12 months’ imprisonment.
The public is reminded that it is an offence under the Medicines (Cosmetic Products) Regulations, 2007 to import and market cosmetic products in the local market without a Cosmetic Product Notification Acknowledgement Letter issued by the authority, where the penalty for contravening these regulations upon conviction, is a fine not exceeding BND5,000, imprisonment for a term not exceeding two years or both.